About AHP
Acute hepatic porphyria (AHP) is a rare, genetic disease characterized by debilitating, potentially life-threatening attacks1,2
There are 4 types of AHP1,3
- Acute Intermittent Porphyria (AIP)~80% of all AHP cases are AIP1
- Variegate Porphyria (VP)
- Hereditary Coproporphyria (HCP)
- ALAD-Deficiency Porphyria (ADP)
ALAD=delta-aminolevulinic acid dehydratase.
AHP attacks can be unpredictable, severe, and progressive1
- AHP can affect anyone, but is most commonly seen in women of childbearing age3
- Attacks generally last 3 to 7 days, but recovery can take longer1
- Severe attacks can progress to respiratory failure or paralysis, leading to temporary or permanent disability2,4
- In 1 study, patients with recurrent attacks averaged 5 hospitalizations in a 12-month period*5
- Some patients face a higher risk of long-term complications, such as hypertension, chronic kidney disease (CKD), hepatocellular carcinoma (HCC), depression, and anxiety4,5
*Results from EXPLORE, a 12-month prospective natural history study of 112 patients who experienced recurrent AHP attacks (≥3 attacks/year) or received prophylactic treatment. Attacks were defined as those requiring increased pain medication or carbohydrate intake, hemin administration, and/or hospitalization for signs and symptoms of AHP.5
"The pain in my abdomen felt like being stabbed with hot knives."
—Donna, an Alnylam Patient Ambassador
Common signs and symptoms of an AHP attack†2,6,7
SEVERE, DIFFUSE ABDOMINAL PAIN6,7
AUTONOMIC
Nervous System6,7
- Nausea/Vomiting
- Constipation
- Tachycardia
- Systemic arterial hypertension
CENTRAL
Nervous System2,6,7
- Seizures
- Anxiety
- Mental status changes
CUTANEOUS7
- Skin lesions on
sun-exposed areas (Cutaneous symptoms primarily occur in HCP and VP)
PERIPHERAL
Nervous System6,7
- Limb weakness
or pain - Peripheral neuropathy
OTHER
Common AHP Symptoms7,8
- Hyponatremia
- Dark, reddish urine
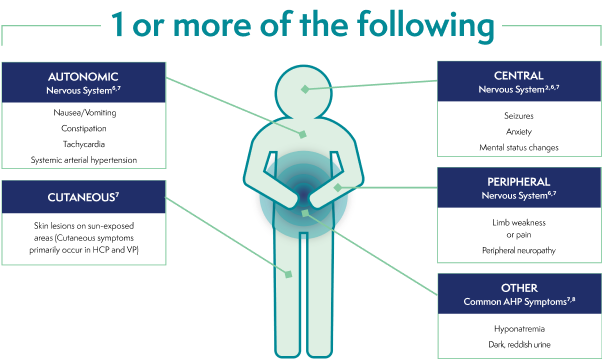
†These are not all the possible signs and symptoms of an AHP attack.
AHP=acute hepatic porphyria; HCP=hereditary coproporphyria; VP=variegate porphyria.
>90% of patients report abdominal pain during AHP attacks6
AHP can go undiagnosed for many years3,9
Because of overlapping symptoms, patients with AHP are often misdiagnosed with other diseases, such as6,10,11:
Gastrointestinal disorders
- Acute gastroenteritis
- with vomiting
- Irritable bowel syndrome (IBS)
- Hepatitis

Gynecological disorder
- Endometriosis
Neurological/neuropsychiatric disorders
- Fibromyalgia
- Guillain-Barré syndrome
- Psychosis

Acute abdomen conditions
- Appendicitis
- Cholecystitis
- Peritonitis
- Pancreatitis
- Intestinal occlusion
Explore the journeys of real AHP patients on GIVLAARI® (givosiran)
Every patient with AHP has a unique story to tell. Discover the journeys to diagnosis and beyond for real patients with AHP.
See Their StoriesReferences: 1. Simon A, Pompilus F, Querbes W, et al. Patient. 2018;11:527-537. 2. Puy H, Gouya L, Deybach JC. Lancet. 2010;375:924-937. 3. Bissell DM, Anderson KE, Bonkovsky HL. N Engl J Med. 2017;377:862-872. 4. Neeleman RA, Wagenmakers MAEM, Koole-Lesuis RH, et al. J Inherit Metab Dis. 2018;41:809-817. 5. Gouya L, Ventura P, Balwani M, et al. Hepatology. 2020;71(5):1546-1558. 6. Ventura P, Cappellini MD, Biolcati G, Guida CC, Rocchi E; Gruppo Italiano Porfiria (GrIP). Eur J Intern Med. 2014;25:497-505. 7. Anderson KE, Bloomer JR, Bonkovsky HL, et al. Ann Intern Med. 2005;142:439-450. 8. Balwani M, Wang B, Anderson KE, et al; Porphyrias Consortium of the Rare Diseases Clinical Research Network. Hepatology. 2017;66:1314-1322. 9. Anderson KE. Mol Genet Metab. 2019;128:219-227. 10. Ko JJ, Murray S, Merkel M, et al. Poster presented at: American College of Gastroenterology Annual Scientific Meeting; October 5-10, 2018; Philadelphia, PA. 11. Alfadhel M, Saleh N, Alenazi H, Baffoe-Bonnie H. Neuropsychiatr Dis Treat. 2014;10:2135-2137.